The Makeup of Earth’s Air
Most people know that oxygen, makes up about 20% of the earth’s atmosphere at sea level, and that almost all the rest is nitrogen. But did you know there’s an impressive list of other gases in the air we breathe
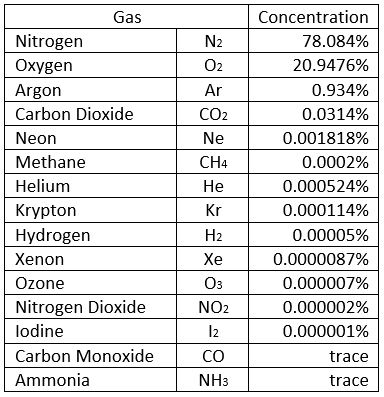
We can consider, for practical purposes, that air is made up of five gases: nitrogen, oxygen, argon, carbon dioxide, and water vapor. But because water vapor is a variable, this table omits it, water vapor generally makes up 1-3% of atmospheric air, by volume, and can be as high as 5%. Which means that, even on a ‘dry’ day, it pushes argon out of third place!
There are numerous reasons why the volumetric concentrations of these gases are important. If oxygen level drops in the air we’re breathing, human activity is impaired. Exhaustion without physical exertion will occur at 12-15%. Your lips turn blue at 10%. Exposure to oxygen levels of 8% or below are fatal within minutes.
But here at EXAIR we care about how compressed air can be used efficiently to better your process!
Any of our products are capable of discharging a fluid, but they’re specifically designed for use with compressed air – in basic grade school science terms, they convert the potential energy of air under compression into kinetic energy in such a way as to entrain a large amount of air from the surrounding environment. This is important to consider for a couple of reasons:
- Anything that’s in your compressed air supply is going to get on the part you’re blowing off with that Super Air Nozzle, the material you’re conveying with that Line Vac, or the electronics you’re cooling with that Cabinet Cooler System. That includes water…which can condense from the water vapor at several points along the way from your compressor’s intake, through its filtration and drying systems, to the discharge from the product itself.
- Sometimes, a user is interested in blowing a purge gas (commonly nitrogen or argon) – but unless it’s in a isolated environment (like a closed chamber) purged with the same gas, most of the developed flow will simply be room air.
Another consideration of air make up involves EXAIR Gen4 Static Eliminators. They work on the Corona discharge principle: a high voltage is applied to a sharp point, and any gas in the vicinity of that point is subject to ionization – loss or gain of electrons in their molecules’ outer valences, resulting in a charged particle. The charge is positive if they lose an electron, and negative if they gain one. Of the two gases that make up almost all of our air, oxygen has the lowest ionization energy in its outer valence, making it the easier to ionize than nitrogen. You can certainly supply a Gen4 Static Eliminator with pure nitrogen if you wish, but the static dissipation rate may be lesser.
If you want to learn more about the compressed air or any of our point of use compressed air products, you can contact an Application Engineer. We will be happy to help you.
Jordan Shouse
Application Engineer
Send me an email
Find us on the Web
Like us on Facebook
Twitter: @EXAIR_JS
Air photo courtesy of Barney Moss Creative Commons License


